ISO 17025 for Testing and Calibration Labs: Ensuring Quality and Competence
In the realm of testing and calibration laboratories, adhering to international standards is crucial to ensure accurate and reliable results. One such standard is ISO 17025. In this article, we will explore what ISO 17025 entails and why it is essential for testing and calibration labs.
What is ISO 17025 Standards?
ISO 17025 is an internationally recognized standard that sets out the general requirements for the competence, impartiality, and consistent operation of testing and calibration laboratories. It provides guidelines and criteria for laboratories to demonstrate their technical competence and proficiency in producing accurate and reliable test results.
What is ISO 17025 and What is its Objective or Purpose?
ISO 17025 serves as a benchmark for testing and calibration labs to ensure the quality and reliability of their services. The standard’s primary objective is to establish confidence in the laboratory’s competence, impartiality, and consistent performance. Let’s delve deeper into the key objectives and purposes of ISO 17025:
1. Ensuring Technical Competence
ISO 17025 emphasizes the need for testing and calibration laboratories to possess the necessary technical competence to carry out their activities effectively. This includes having skilled personnel, adequate infrastructure, calibrated equipment, validated test methods, and reliable quality assurance procedures. By adhering to ISO 17025, labs can enhance their technical capabilities and ensure accurate and reliable testing and calibration processes.

2. Demonstrating Impartiality and Independence
Independence and impartiality are vital principles in testing and calibration. ISO 17025 requires labs to demonstrate freedom from commercial, financial, and other pressures that may compromise their impartiality. This ensures that test results and calibrations are objective, unbiased, and free from conflicts of interest. Clients and stakeholders can have confidence in the integrity and reliability of the lab’s services.
3. Establishing Quality Management System
ISO 17025 places great importance on implementing a robust quality management system (QMS) within the laboratory. This includes document control, record keeping, equipment maintenance, staff training, internal audits, and management review. By adopting a well-defined QMS, labs can ensure consistent adherence to quality procedures, continual improvement, and customer satisfaction.
4. Promoting International Recognition and Acceptance
ISO 17025 is an internationally recognized standard. Obtaining accreditation to ISO 17025 enhances the credibility and acceptance of a laboratory’s test results and calibrations both nationally and internationally. It facilitates the acceptance of test reports across borders and eliminates the need for retesting or recalibration, saving time and resources for clients
quality management system (QMS) within the laboratory. This includes document control, record keeping, equipment maintenance, staff training, internal audits, and management review. By adopting a well-defined QMS, labs can ensure consistent adherence to quality procedures, continual improvement, and customer satisfaction.
4. Promoting International Recognition and Acceptance
ISO 17025 is an internationally recognized standard. Obtaining accreditation to ISO 17025 enhances the credibility and acceptance of a laboratory’s test results and calibrations both nationally and internationally. It facilitates the acceptance of test reports across borders and eliminates the need for retesting or recalibration, saving time and resources for clients.
What are testing and calibration laboratories?
Testing and calibration laboratories are specialized facilities that conduct a wide range of activities to ensure the accuracy, reliability, and validity of measurements and testing results. These laboratories play a critical role in various industries, including manufacturing, healthcare, environmental monitoring, food and beverage, construction, and more. Here’s a closer look at testing and calibration laboratories:
Testing Laboratories: Testing laboratories are equipped with advanced instrumentation and expertise to perform tests on various materials, products, or samples. These tests can encompass a wide range of parameters, such as physical, chemical, mechanical, electrical, and biological properties. Testing labs conduct experiments and analyses to determine the characteristics, performance, quality, safety, and compliance of products, materials, or samples. The results of these tests provide essential information for decision-making, quality control, research and development, product certification, and regulatory compliance
Calibration Laboratories: Calibration laboratories focus on ensuring the accuracy and reliability of measuring instruments and equipment. They perform calibration processes to verify and adjust the measurements of instruments to a known standard. Calibration involves comparing the instrument’s output with a reference standard of higher accuracy to assess any deviations or errors. The calibration process helps establish traceability and ensures that measuring instruments are operating within acceptable tolerances. Calibration labs play a crucial role in maintaining the accuracy and consistency of measurement results across different industries, including manufacturing, healthcare, engineering, and scientific research.
Both testing and calibration laboratories are typically operated by qualified professionals, scientists, engineers, or technicians with expertise in their respective fields. These laboratories follow standardized protocols, testing methods, and quality assurance procedures to ensure the accuracy, reliability, and consistency of their testing and calibration processes. Accreditation to international standards, such as ISO 17025, demonstrates their competence, impartiality, and adherence to recognized best practices in testing and calibration.
Why is it important to calibrate your instruments with ISO 17025 Accredited lab?
Calibrating instruments with an ISO 17025 accredited laboratory is important for several reasons:
- Accurate and Reliable Measurements: ISO 17025 accredited labs adhere to stringent quality standards and follow internationally recognized calibration procedures. They have well-defined processes and use traceable reference standards to calibrate instruments. By calibrating your instruments with such labs, you can ensure that your measurements are accurate, reliable, and traceable to national or international standards.
- Compliance with Regulations and Standards: Many industries and regulatory bodies require calibration certificates from accredited labs as proof of instrument accuracy and compliance. Using an ISO 17025 accredited lab ensures that your calibration certificates are recognized and accepted by regulatory agencies, customers, and other stakeholders. It helps demonstrate your commitment to quality, regulatory compliance, and meeting industry standards.
- Consistent and Comparable Results: Calibration by an accredited lab ensures consistency and comparability of measurement results. Accredited labs follow standardized procedures, control environmental factors, and employ trained personnel to minimize measurement uncertainties. This allows for consistent and repeatable measurements, enabling you to compare results over time and across different instruments or laboratories.
- Confidence and Credibility: ISO 17025 accreditation signifies that a laboratory has undergone rigorous evaluation by an independent accreditation body. It demonstrates the lab’s technical competence, adherence to quality management systems, and commitment to impartiality. Calibrating your instruments with an accredited lab gives you confidence in the accuracy of your measurements and enhances your credibility with customers, regulatory agencies, and other stakeholders.
- International Recognition: ISO 17025 accreditation is recognized globally, facilitating acceptance of calibration certificates across borders. If your business operates internationally or deals with customers or partners from different countries, using an accredited lab ensures that your calibration certificates are widely accepted, eliminating the need for additional calibration or verification.
- Risk Mitigation: Accurate measurements are crucial for decision-making, quality control, and product conformity. Using instruments that are calibrated by an accredited lab reduces the risk of making incorrect decisions, faulty products, or non-compliance with regulations. It helps minimize the potential for errors, product recalls, customer complaints, and financial losses.
calibrating your instruments with an ISO 17025 accredited lab ensures accurate and reliable measurements, compliance with regulations and standards, consistent and comparable results, confidence and credibility, international recognition, and effective risk mitigation. It is a proactive step towards maintaining quality, meeting regulatory requirements, and demonstrating your commitment to excellence in measurement and instrumentation.
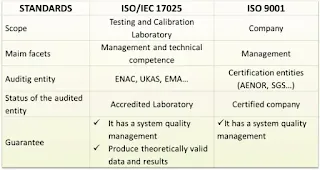
What is the difference between the ISO 9001 and the ISO 17025?
|
Aspect |
ISO 9001 |
ISO 17025 |
|
Definition |
A standard for Quality Management Systems (QMS). |
A standard for the competence of testing and calibration laboratories. |
|
Purpose |
Ensures organizations meet customer and stakeholder needs through effective quality management. |
Ensures labs produce valid and reliable results by setting out criteria for lab operation and competence. |
|
Scope |
Broad and applicable to any organization, regardless of its type, size, or the product/service it provides. |
Specific to laboratories that conduct testing and calibration. |
|
Main Focus |
Focuses on management processes and continual improvement. |
Focuses on technical competence, validity of results, and impartiality of laboratory operations. |
|
Certification |
Organizations can obtain ISO 9001 certification by demonstrating conformity to the standard. |
Laboratories can obtain ISO 17025 accreditation by demonstrating technical competence and valid results. |
|
Requirements |
Covers a wide range of management principles including leadership, planning, operations, and performance evaluation. |
Covers management requirements (similar to ISO 9001) and technical requirements (specific procedures, environmental conditions, etc.) for labs. |
|
Document Control |
Requires documented information to be maintained and controlled. |
Requires a more detailed approach to documents, including procedures for test methods, calibration standards, and traceability. |
|
Auditing |
Requires internal audits and management review processes. |
Requires both internal and external audits, ensuring consistent and reliable laboratory results. |
|
Continuous Improvement |
Emphasizes the importance of continuous improvement of the QMS. |
Focuses on the continuous improvement of technical practices and results accuracy. |
While both standards are geared towards quality assurance, ISO 9001 is broad and can be applied to any organization looking to improve its quality management systems, whereas ISO 17025 is specific to the technical and operational competence of laboratories.
ISO 9001 is applicable to all types of organizations, including manufacturing, services, healthcare, education, and more. It aims to ensure that organizations consistently deliver products and services that meet customer expectations, comply with applicable regulations, and drive continual improvement in quality performance.
ISO 17025: Testing and Calibration Laboratory Standard: ISO 17025 is a specific standard designed for testing and calibration laboratories. It specifies the general requirements for the competence, impartiality, and consistent operation of these laboratories. ISO 17025 focuses on the technical competency and quality management requirements specific to testing and calibration activities. It covers areas such as personnel competence, calibration procedures, measurement traceability, equipment suitability and calibration, testing methodologies, quality control, reporting of results, and management of non-conformities.
ISO 17025 is applicable to laboratories performing testing, calibration, and sampling activities across various fields, including chemical analysis, biological testing, mechanical testing, environmental monitoring, and more. It aims to ensure that testing and calibration laboratories consistently produce accurate and reliable results, adhere to recognized standards and procedures, and have effective quality management systems in place.
ISO 9001 is a generic quality management system standard applicable to all types of organizations, whereas ISO 17025 is specifically tailored for testing and calibration laboratories, focusing on technical competency and quality management specific to laboratory operations
What are the benefits of ISO 17025 accreditation?
Accreditation to ISO 17025, the international standard for testing and calibration laboratories, offers numerous benefits for laboratories and their stakeholders. Here are some key benefits of ISO 17025 accreditation:
- Enhanced Credibility and Reputation: ISO 17025 accreditation demonstrates a laboratory’s technical competence, impartiality, and adherence to recognized standards. It enhances the credibility and reputation of the laboratory, giving confidence to customers, regulatory bodies, and other stakeholders in the accuracy and reliability of the laboratory’s testing and calibration services.
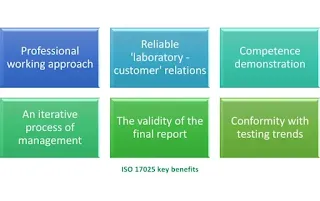
- Global Recognition and Acceptance: ISO 17025 accreditation is recognized and accepted internationally. Accredited laboratories enjoy wider acceptance of their test reports and calibration certificates across borders, eliminating the need for additional testing or calibration by customers or regulatory agencies. This recognition opens doors to new markets and facilitates international collaborations.
- Improved Quality Management System: ISO 17025 accreditation requires laboratories to establish and maintain a robust quality management system. Through accreditation, laboratories are encouraged to implement best practices in quality management, including document control, equipment calibration, competence assessment, proficiency testing, internal audits, and corrective actions. This focus on quality management leads to improved operational efficiency, consistency, and customer satisfaction.
- Demonstrated Technical Competence: Accreditation to ISO 17025 verifies a laboratory’s technical competence in specific fields of testing and calibration. It ensures that the laboratory has the necessary infrastructure, equipment, procedures, and personnel with appropriate qualifications and expertise to perform accurate and reliable measurements. Accreditation assures stakeholders that the laboratory’s results are traceable and meet recognized standards.
- Enhanced Data Accuracy and Reliability: ISO 17025 accreditation promotes the use of validated test methods, calibrated equipment, and well-documented procedures, leading to accurate and reliable test results. The accreditation process includes proficiency testing and inter-laboratory comparisons, which further validate the laboratory’s measurement capabilities and ensure consistency in results. Accurate and reliable data are essential for informed decision-making, quality control, and regulatory compliance.
- Compliance with Regulatory Requirements: Many regulatory bodies require testing and calibration to be performed by accredited laboratories. ISO 17025 accreditation helps laboratories meet regulatory requirements, ensuring that their services comply with relevant standards and regulations. It provides confidence to regulators that the laboratory operates in a competent and reliable manner.
- Continuous Improvement Culture: ISO 17025 accreditation promotes a culture of continuous improvement within the laboratory. Regular internal audits, management reviews, and corrective action processes encourage laboratories to identify areas for improvement, address non-conformities, and implement preventive measures. This commitment to continual improvement leads to increased efficiency, effectiveness, and customer satisfaction.
ISO 17025 accreditation offers benefits such as enhanced credibility and reputation, global recognition, improved quality management system, demonstrated technical competence, accurate and reliable data, compliance with regulations, and a culture of continuous improvement. Accreditation provides assurance to customers, regulatory bodies, and stakeholders that a laboratory operates at a high standard of quality and technical competence.
Is ISO/IEC 17025 Mandatory?
ISO/IEC 17025 is not mandatory by default, but it may be required or recommended in certain situations. The applicability of ISO/IEC 17025 depends on various factors, including regulatory requirements, customer demands, industry norms, and contractual obligations. Here are a few scenarios where ISO/IEC 17025 accreditation may be mandatory or highly beneficial:
- Regulatory Compliance: Some industries, such as healthcare, environmental testing, food safety, and pharmaceuticals, have specific regulations that mandate compliance with ISO/IEC 17025 or similar standards. In these cases, accreditation to ISO/IEC 17025 becomes a legal or regulatory requirement for operating in the industry.
- Contractual Obligations: Customers or regulatory bodies may require suppliers or testing/calibration laboratories to have ISO/IEC 17025 accreditation as a condition for providing services or products. This is common when dealing with government contracts, large organizations, or industries that prioritize quality and accuracy.
- International Recognition: ISO/IEC 17025 accreditation provides international recognition and acceptance of a laboratory’s technical competence and quality management systems. If a laboratory wants to demonstrate its capabilities to international clients or participate in global collaborations, ISO/IEC 17025 accreditation can greatly enhance its credibility and market competitiveness.
- Improved Quality and Competence: Even if ISO/IEC 17025 is not mandatory, many laboratories voluntarily pursue accreditation to improve their quality management systems, enhance technical competence, and provide assurance of reliable and accurate testing/calibration results. Accreditation helps laboratories to establish standardized procedures, implement best practices, and gain customer confidence.
It’s important to consider the specific requirements of your industry, regulatory environment, and customer expectations to determine whether ISO/IEC 17025 accreditation is mandatory or highly beneficial for your laboratory. Consulting with industry associations, regulatory bodies, or accreditation bodies can provide further guidance on the applicability and advantages of ISO/IEC 17025 in your specific context.
What are the Requirements of testing and calibration laboratory for ISO 17025 ?
The ISO/IEC 17025 standard specifies the requirements for the competence and consistent operation of testing and calibration laboratories. Here are the key requirements outlined in ISO/IEC 17025 for testing and calibration laboratories:
- Organization and Management:
-
- Documented policies, procedures, and organizational structure.
- Clearly defined roles, responsibilities, and authorities for personnel.
- Management commitment to impartiality, confidentiality, and continual improvement.
- Quality Management System (QMS):
-
- Establish and maintain a comprehensive quality management system.
- Document control processes to ensure the availability of up-to-date documents and records.
- Implement processes for managing non-conformities, corrective actions, and preventive actions.
- Conduct management reviews to evaluate the effectiveness and improvement of the QMS.
- Personnel Competence:
-
- Define the competence requirements for personnel based on education, training, and experience.
- Ensure that personnel are trained and competent to perform their assigned tasks.
- Maintain records of personnel competence, including training, qualifications, and experience.
- Testing and Calibration Processes:
-
- Implement appropriate testing and calibration methods and procedures.
- Ensure the availability and proper calibration of measurement equipment.
- Validate and verify methods for their intended use.
- Ensure traceability of measurements to national or international standards.
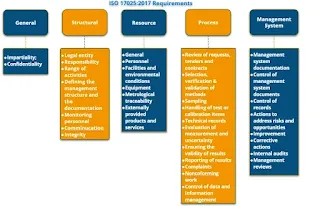
- Quality Assurance:
-
- Implement quality control procedures to monitor the validity and accuracy of test/calibration results.
- Establish proficiency testing programs and participation in interlaboratory comparisons.
- Maintain records of quality control activities and results.
- Conduct internal audits to assess compliance with the standard and the QMS.
- Reporting of Results:
-
- Ensure accurate and complete reporting of test/calibration results.
- Include all relevant information, such as measurement uncertainty, traceability, and limitations.
- Maintain records of test/calibration results, including raw data, calculations, and observations.
- Facilities and Environmental Conditions:
-
- Provide suitable facilities, equipment, and environmental conditions for testing and calibration activities.
- Control environmental factors that could affect the validity of test/calibration results, such as temperature, humidity, and lighting.
It’s important to note that the requirements of ISO/IEC 17025 may vary based on the specific field of testing or calibration and the scope of laboratory activities. Laboratories seeking accreditation should carefully review the complete ISO/IEC 17025 standard and consult with accreditation bodies to ensure compliance with all relevant requirements.
How to get ISO 17025 accredited?
Getting ISO/IEC 17025 accreditation involves a series of steps and processes. Here is a general overview of the typical path to achieve ISO/IEC 17025 accreditation for a testing or calibration laboratory:
- Familiarize Yourself with ISO/IEC 17025: Read and understand the requirements of the ISO/IEC 17025 standard. Familiarize yourself with the specific clauses, technical competence requirements, and management system elements outlined in the standard.
- Gap Analysis and Preparation: Conduct a thorough gap analysis of your laboratory’s current practices and the requirements of ISO/IEC 17025. Identify areas where your laboratory needs to improve to meet the standard’s requirements. Develop an implementation plan and establish a project team responsible for the accreditation process.
- Documenting the Quality Management System: Develop and document your laboratory’s quality management system (QMS) based on the requirements of ISO/IEC 17025. This includes creating policies, procedures, and work instructions to address each clause of the standard. Document control processes and establish a system for managing records.
- Implementation of the QMS: Implement the documented QMS throughout your laboratory. Ensure that personnel are trained on the new procedures and processes. Implement mechanisms for monitoring, measurement, and analysis of the QMS’s effectiveness.
- Internal Audits and Corrective Actions: Conduct internal audits to assess the compliance of your laboratory’s operations with the requirements of ISO/IEC 17025. Identify any non-conformities and take corrective actions to address them. Continually monitor and improve the effectiveness of your QMS.
- Engage an Accreditation Body: Select an accreditation body that is recognized and approved for ISO/IEC 17025 accreditation. Contact the accreditation body and express your interest in seeking accreditation. They will provide you with the necessary information and guidance on their specific accreditation process.
- Assessment and Evaluation: The accreditation body will conduct an assessment of your laboratory. This typically involves a detailed evaluation of your QMS, laboratory facilities, equipment, personnel competence, and proficiency testing. The assessment may include on-site visits, interviews, and examination of records and procedures.
- Accreditation Decision: Based on the assessment, the accreditation body will make a decision regarding your laboratory’s accreditation. If your laboratory meets the requirements of ISO/IEC 17025, you will be granted accreditation.
- Ongoing Compliance and Surveillance: After accreditation, your laboratory will undergo periodic surveillance audits by the accreditation body to ensure ongoing compliance with ISO/IEC 17025. These audits typically occur annually or as determined by the accreditation body.
It’s important to note that the accreditation process can vary depending on the specific accreditation body and the region in which you are seeking accreditation. It is recommended to contact the chosen accreditation body and follow their specific guidelines and requirements throughout the accreditation process.
What does ISO 17025 accreditation mean?
ISO/IEC 17025 accreditation signifies that a testing or calibration laboratory has been assessed and found to meet the requirements of the ISO/IEC 17025 standard. It is a globally recognized accreditation that demonstrates the laboratory’s technical competence and ability to produce reliable and accurate results.
Here are the key aspects and implications of ISO/IEC 17025 accreditation:
- Technical Competence: ISO/IEC 17025 accreditation confirms that the laboratory possesses the necessary technical competence to perform specific testing or calibration activities. It assures customers and stakeholders that the laboratory has the expertise, skills, and resources to conduct accurate and reliable measurements and evaluations.
- Quality Management Systems: ISO/IEC 17025 requires laboratories to establish and maintain a robust quality management system (QMS). Accreditation verifies that the laboratory’s QMS conforms to the standard’s requirements, including document control, record keeping, competency management, equipment calibration, and measurement traceability.
- International Recognition: ISO/IEC 17025 is globally recognized and accepted as the benchmark for testing and calibration laboratory accreditation. Accreditation provides international recognition of the laboratory’s technical competence, enhancing its credibility and facilitating acceptance of test/calibration results across national borders.
- Improved Confidence and Credibility: ISO/IEC 17025 accreditation instills confidence in customers and stakeholders regarding the laboratory’s ability to deliver accurate and reliable results. It demonstrates the laboratory’s commitment to quality, accuracy, and continuous improvement, which can positively impact customer satisfaction and enhance the laboratory’s reputation.
- Compliance with Regulations and Standards: ISO/IEC 17025 accreditation helps laboratories meet regulatory and industry-specific requirements. It ensures that the laboratory operates in accordance with established standards, regulations, and best practices, addressing the needs of sectors such as healthcare, environmental testing, pharmaceuticals, and more.
- Competitive Advantage: ISO/IEC 17025 accreditation can provide a competitive advantage for a laboratory. Accredited laboratories are often preferred by customers, as it assures them of reliable and accurate testing/calibration services. Accreditation can lead to increased business opportunities, customer trust, and market recognition.
It’s important to note that ISO/IEC 17025 accreditation is not a one-time achievement. Accredited laboratories are subject to periodic surveillance audits to ensure ongoing compliance with the standard. This ensures that the laboratory maintains its technical competence and upholds the quality management systems required by ISO/IEC 17025.
How many clauses are there in ISO 17025:2017?
ISO/IEC 17025:2017, the standard for testing and calibration laboratories, consists of 10 clauses. These clauses cover the various requirements that laboratories must fulfill to demonstrate technical competence and maintain a reliable quality management system. Here is a list of the 10 clauses in ISO/IEC 17025:2017:
- Scope
- Normative References
- Terms and Definitions
- General Requirements
- Structural Requirements
- Resource Requirements
- Process Requirements
- Management System Requirements
- Technical Records
- Management System Records
Each of these clauses outlines specific requirements that laboratories must address to achieve and maintain ISO/IEC 17025 accreditation. The standard covers various aspects of laboratory operations, including management commitment, personnel competence, equipment calibration, measurement traceability, handling of test items, and reporting of test results, among others. The requirements are designed to ensure the technical competence and consistency of laboratory operations to produce reliable and accurate test/calibration results.
How to get ISO 17025 Certification in Nepal?
Obtaining ISO/IEC 17025 certification in Nepal involves a series of steps to demonstrate compliance with the standard’s requirements. Here is a general outline of the process:
- Familiarize Yourself with the Standard: Read and understand the requirements of ISO/IEC 17025:2017. Familiarize yourself with the clauses, technical competence requirements, and management system elements outlined in the standard.
- Conduct a Gap Analysis: Evaluate your laboratory’s current practices against the requirements of ISO/IEC 17025. Identify areas where your laboratory needs to improve to meet the standard’s requirements.
- Develop a Quality Management System (QMS): Establish and document your laboratory’s quality management system based on the requirements of ISO/IEC 17025. Develop policies, procedures, and work instructions to address each clause of the standard. Implement document control processes and establish a system for managing records.
- Implement the QMS: Implement the documented QMS throughout your laboratory. Ensure that personnel are trained on the new procedures and processes.
- Internal Audits and Corrective Actions: Conduct internal audits to assess the compliance of your laboratory’s operations with the requirements of ISO/IEC 17025. Identify any non-conformities and take corrective actions to address them. Continually monitor and improve the effectiveness of your QMS.
- Select an Accreditation Body: Choose an accreditation body that is recognized and approved for ISO/IEC 17025 accreditation. In Nepal, you can contact the Nepal Bureau of Standards and Metrology (NBSM), which is the national accreditation body.
- Submit Application for Accreditation: Submit your application for ISO/IEC 17025 accreditation to the chosen accreditation body. Provide all necessary documentation and evidence of your laboratory’s compliance with the standard.
- Assessment and Evaluation: The accreditation body will conduct an assessment of your laboratory. This typically involves a detailed evaluation of your QMS, laboratory facilities, equipment, personnel competence, and proficiency testing. The assessment may include on-site visits, interviews, and examination of records and procedures.
- Accreditation Decision: Based on the assessment, the accreditation body will make a decision regarding your laboratory’s accreditation. If your laboratory meets the requirements of ISO/IEC 17025, you will be granted accreditation.
- Ongoing Compliance and Surveillance: After accreditation, your laboratory will undergo periodic surveillance audits by the accreditation body to ensure ongoing compliance with ISO/IEC 17025. These audits typically occur annually or as determined by the accreditation body.
It is essential to follow the specific guidelines and requirements of the Nepal Bureau of Standards and Metrology or the chosen accreditation body throughout the accreditation process. The process duration may vary, depending on the complexity of your laboratory’s operations and the accreditation body’s procedures.
What should be the of accuracy as per ISO 17025?
ISO/IEC 17025, the standard for testing and calibration laboratories, does not prescribe specific accuracy requirements for measurements. Instead, it focuses on ensuring the competency and reliability of the laboratory’s testing and calibration processes. However, ISO/IEC 17025 emphasizes the need for laboratories to establish and maintain measurement traceability, estimate measurement uncertainty, and implement quality control measures.
The accuracy of measurements depends on several factors, including the specific test or calibration being performed, the equipment and methods used, and the requirements of the customers or applicable standards. Accuracy requirements are typically determined by industry-specific standards, regulations, or customer specifications.
To meet the requirements of ISO/IEC 17025, laboratories must:
- Establish measurement traceability: Laboratories should have a documented process to demonstrate the traceability of their measurements to national or international standards. This ensures that the measurements are based on recognized reference materials and methods.
- Estimate measurement uncertainty: Laboratories should estimate and report the uncertainty associated with their measurements. Measurement uncertainty quantifies the range within which the true value of the measured quantity is likely to lie. It reflects the combined effects of various sources of errors and provides an indication of the measurement’s reliability.
- Implement quality control measures: Laboratories must establish procedures for quality control to monitor and control the accuracy of their measurements. This may involve using reference materials, participating in proficiency testing, conducting internal quality control checks, and maintaining suitable calibration of equipment.
It is important for laboratories to understand the accuracy requirements specific to their field of testing or calibration. Compliance with ISO/IEC 17025 involves demonstrating technical competency and adherence to best practices, ensuring that measurements are performed accurately and reliably within the laboratory’s defined scope.
ISO/IEC 17025 does not provide specific accuracy requirements but emphasizes the need for measurement traceability, estimation of measurement uncertainty, and implementation of quality control measures to ensure the accuracy and reliability of test and calibration results. The accuracy requirements are determined by industry-specific standards, regulations, and customer specifications.
Conclusion
In conclusion, ISO/IEC 17025:2017 is a vital standard for testing and calibration labs. It ensures technical competence, reliable results, and quality management. Accreditation to this standard enhances credibility and demonstrates commitment to accuracy and reliability. Adopting ISO/IEC 17025 improves processes, meets customer needs, and maintains quality improvement. It establishes a strong foundation for excellence in testing and calibration.
Frequently Asked Questions:
What is ISO 17025?
ISO 17025 is an international standard that sets requirements for the competence, impartiality, and consistent operation of testing and calibration laboratories. It ensures that laboratories perform accurate and reliable tests or calibrations, providing confidence in their results.
What is ISO 17025 PDF?
ISO 9001:2008 was the previous version of the ISO 9001 standard. It was replaced by ISO 9001:2015 and has since been outdated. Organizations that were certified under ISO 9001:2008 were required to transition to the new version.
ISO 17025 PDF refers to the digital format of the ISO/IEC 17025 standard document. It is a downloadable version of the standard that can be accessed, viewed, and used electronically for reference and implementation in laboratories seeking ISO 17025 accreditation.
- ISO 17025: ISO 17025 is an international standard that specifies the general requirements for the competence of testing and calibration laboratories. It provides guidelines for laboratories to demonstrate their ability to produce accurate and reliable results.
- ISO 17025:2005: ISO 17025:2005 was the previous version of the standard, specifying the requirements for laboratory competence. It has been replaced by ISO/IEC 17025:2017.
- ISO 17025 Checklist: An ISO 17025 checklist is a tool used to assess a laboratory’s compliance with the requirements of the ISO 17025 standard. It helps ensure that all necessary aspects are covered during the audit or assessment process.
- ISO 17025 Consultant: An ISO 17025 consultant is a professional who provides guidance and support to laboratories seeking compliance with ISO 17025. They help implement the necessary processes and procedures to meet the standard’s requirements.
- ISO 17025 Laboratory Management System: ISO 17025 laboratory management system refers to the set of processes, procedures, and practices adopted by a laboratory to ensure quality and competence in testing and calibration activities.
- ISO 17025 Quality Management System: ISO 17025 quality management system refers to the framework used by laboratories to ensure that their operations meet the requirements of the standard, leading to accurate and reliable test results.
- ISO 17025 Standard: The ISO 17025 standard, officially known as ISO/IEC 17025:2017, specifies the requirements for the competence of testing and calibration laboratories. It covers areas such as personnel, equipment, testing methods, and quality management.
- ISO 17025 Training Course: An ISO 17025 training course is designed to educate laboratory personnel and management about the requirements of the standard and how to implement them effectively in their daily operations.
- Audit Checklist for ISO 17025:2017: An audit checklist for ISO 17025:2017 is a tool used during the audit process to verify a laboratory’s compliance with the specific clauses and requirements of the updated standard.
- ISO 17025:2005 Detailed Explanation of Clauses with Examples: For detailed explanations of specific clauses with examples, it is recommended to refer to official ISO documents, training materials, or resources provided by recognized accreditation bodies.
- ISO 17025:2005 General Requirements: ISO 17025:2005’s general requirements covered the need for laboratories to demonstrate competence in conducting specific tests or calibrations, ensuring validity, accuracy, and confidentiality of results.
- ISO 17025:2017 Explanation of 6.2.5: ISO 17025:2017 clause 6.2.5 deals with “Equipment.” It outlines the requirements for laboratories to ensure that the equipment used for testing and calibration activities is suitable, calibrated, and maintained.
- ISO 17025 Scope of Accreditation: The ISO 17025 scope of accreditation refers to the specific areas and types of testing or calibration activities for which a laboratory is accredited and recognized as competent.
- ISO 17025 PowerPoint Slides: ISO 17025 PowerPoint slides may refer to ISO 17025: ISO 17025 is an international standard that specifies the general requirements for the competence of testing and calibration laboratories. It provides guidelines for laboratories to demonstrate their ability to produce accurate and reliable results.
In order to maintain a seamless and efficient ISO certification process, partnering with a trusted ISO consultant is crucial. At Quality Management System Nepal Pvt. Ltd., we are committed to providing your organization with expert guidance and support, ensuring a cost-effective and successful ISO implementation journey. As the leading ISO System Certification body in Nepal, we offer a comprehensive range of certification services tailored to your organization’s needs.
Our dedicated team is ready to provide your organization with customized solutions and expert assistance. If you have any queries or are ready to embark on your ISO certification journey, feel free to contact us at 9840525565 for a free consultation on our ISO certification services. Trust Quality Management System Nepal Pvt. Ltd. to be your partner in achieving excellence and compliance.